(WAND) - Additional medications used to treat high blood pressure are being recalled by the Food and Drug Administration.
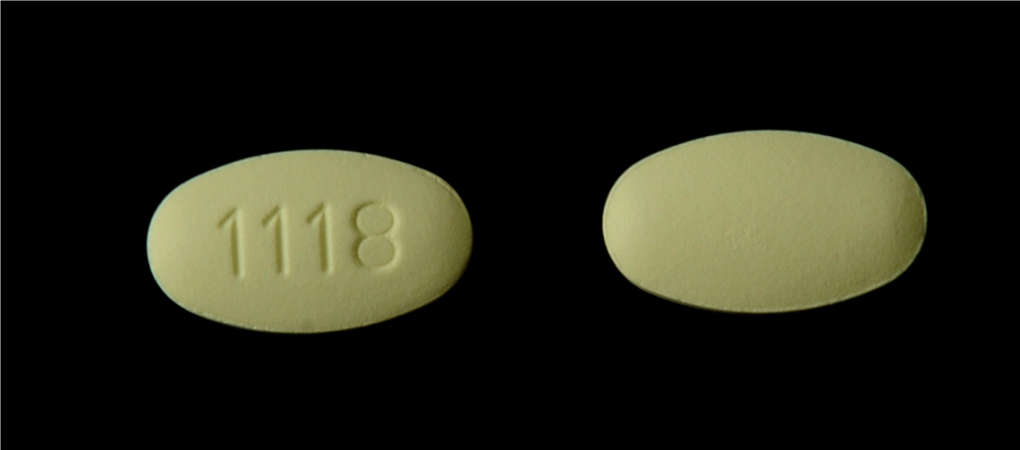
According to the FDA, certain lots of Losartan Potassium tablets are being recalled. The medication is used to treat hypertension, hypertensive patients with Left Ventricular Hypertrophy and nephropathy in Type 2 diabetic patients.
Several lots of the drug found N-Methylnitrosobutyricacid (NMBA), which could potentially cause cancer.
The recall was expanded to include an additional 3 lots of Losartan Potassium Tablets USP and 2 lots of Losartan Potassium/Hydrochlorothiazide Tablets, USP.
According to the FDA, Patients taking Losartan Potassium Tablets USP and Losartan Potassium / Hydrochlorothiazide Tablets, USP should continue taking their medication, as the risk of harm to the patient's health may be higher if the treatment is stopped immediately without any alternative treatment. Patients should contact their pharmacist or physician who can advise them about an alternative treatment prior to returning their medication.
Products included in the recall are listed below:
- 13668-409-10 Losartan Potassium Tablets, USP 50mg, 1000 count 4DU2E009 12/31/2020
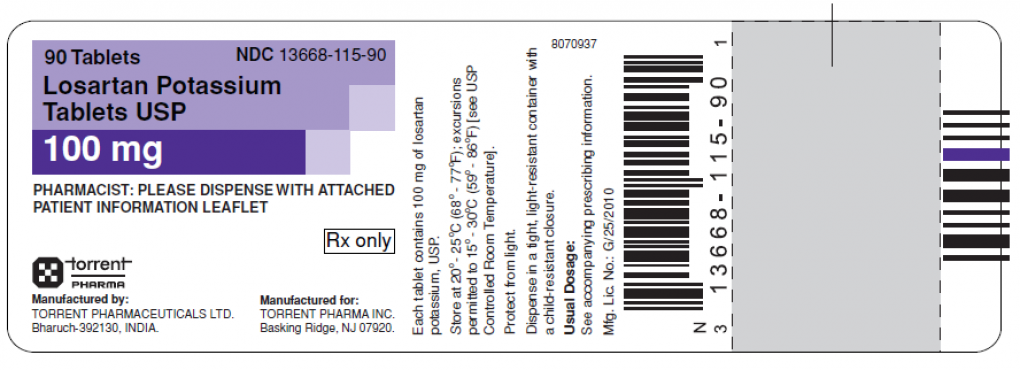
- 13668-115-90 Losartan Potassium Tablets, USP 100mg, 90 count 4DU3E009 12/31/2020
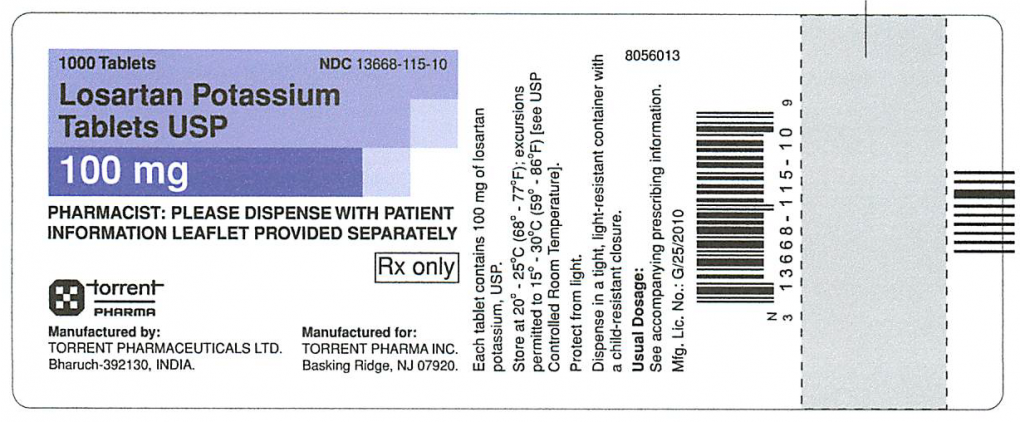
- 13668-115-10 Losartan Potassium Tablets, USP 100mg, 1000 count 4DU3D018 02/28/2021
- 13668-116-90 Losartan Potassium / Hydrochlorothiazide Tablets, USP 50mg/12.5mg, 90 count BEF7D051 11/30/2020
- 13668-118-90 Losartan Potassium / Hydrochlorothiazide Tablets, USP 100mg/25mg, 90 count. 4P04D007 07/31/2020