DECATUR, Ill. (WAND) - Hot, summer weather is coming! And, to understand why it's getting so hot, let us examine the first law of thermodynamics.
The first law of thermodynamics, also known as the law of conservation of energy, states that energy cannot be created or destroyed. Instead, energy can only be transferred or changed from one form to another. This law implies that the total energy remains constant, accounting for energy exchanges through heat and work.
Now, let's discuss compressional heating. This is the process in which a gas or fluid increases in temperature due to compression.
When a substance is compressed—meaning its volume decreases under pressure—its particles are forced closer together, leading to an increase in internal energy and, consequently, temperature. This is exactly what one would expect when following the first law of thermodynamics.
Think of it this way: when you pump air through a small tube into your car tire, the rubber hose becomes hot. These are the air particles being compressed into the tube and the increase in friction releases heat, making the tube feel warm or hot.
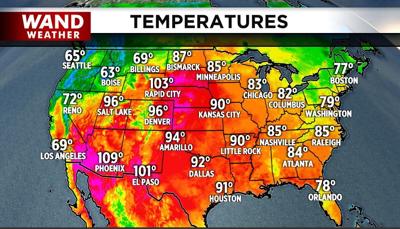
So, what's the bottom line here? We look at the origin of our current air mass arriving from the Great Basin. That air traveled over the Continental Divide and is now moving eastward.
Notice the pink shaded areas of the temperature map that indicate 100+ degrees. These are areas east of the Continental Divide.
So, as the air descends from the mountain tops, the first law of thermodynamics comes into play. The air warms on average by 5.4 degrees per 1,000 feet descent if it's unsaturated (dry); 3.4 degrees if it is saturated (wet).
The air then spills across the Plains toward the Midwest, and the end result is the hottest weather of the year-to-date, starting this weekend.
Copyright 2025. WANDTV. All Rights Reserved.